Year: 2019
-

U DREAM PRODUCT Recall by HEALTH CANADA
Health Canada has tested several U-Dream Lite and U-Dream Full Night herbal sleep-aid products and has found that they contain a substance similar to the prescription drug zopiclone, which may pose serious health risks. Health Canada tested the products after receiving complaints of unusual side effects—such as symptoms of withdrawal and dependence—suggesting that the products…
-
Ebola vaccine
The Ebola virus causes an acute, serious illness which is often fatal if untreated. Ebola virus disease (EVD) known as Ebola haemorrhagic fever, is a rare but severe, often fatal illness in humans. The virus is transmitted to people from wild animals and spreads in the human population through human-to-human transmission via direct contact (through broken…
-
36. Recommendation for Action and Exchange of Information
This step is performed by the pharmacovigilance physician: Based on the level of safety risk or patient impact, recommend action and review recommendations with the product safety board. The recommendations may include any or a combination of the following conclusions: A post-authorisation study to be conducted according to an agreed protocol and submit the final…
-
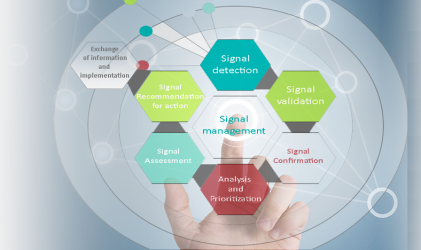
35. Signal Assessment – Evaluation of Risk
The objective of signal assessment is to further evaluate the significance and potential risk of a validated signal so as to identify the need for additional data collection, risk mitigation or minimization activities in a timely manner, or for any regulatory action. The following steps will be performed for signal assessment: Review appropriate internal and…
-

34. Signal prioritisation
Signal prioritisation is the process, continuously performed throughout signal management, which aims to identify those signals suggesting risks with a potential important patients’ or public health impact or which may significantly affect the risk-benefit balance of the medicinal product and thus require urgent attention and management without delay. Below aspects to be consider while prioritizing:…
-

33. Signal validation
Signal validation is the process of evaluating the data supporting the detected signal in order to verify that the available documentation contains sufficient evidence demonstrating the existence of a new potentially causal association, or a new aspect of a known association, and therefore justifies further analysis of the signal. This evaluation should take into account…
-
32. Signal Management – Signal detection
Signal Management Definition: A set of activities performed to determine whether, based on an examination of individual case safety reports (ICSRs), aggregated data from active surveillance systems or studies, scientific literature information or other data sources, there are new risks associated with an active substance or a medicinal product or whether known risks have changed,…
-
31. Safety Signal – Introduction
What is Signal? A signal is essentially a hypothesis of a risk with a medicine with data and arguments that support it, derived from data from one or more of many possible sources. The evidence in a signal is not conclusive (is, in the technical sense, uncertain), and is only an early indication (preliminary), as…
-
30. Periodic Adverse Drug Experience Report (PADER/PAER)
A PADER is a type of aggregate safety report required to be submitted by a sponsor or marketing authorization holder (MAH) to the US Food and Drug Administration (FDA) after obtaining marketing authorization approval as per 314.80 (C) (2) and 600.80 (C)(2) guidelines. PADERs are largely superceded by the new PBRER for hormonised use across…
-
29. Periodic Safety Update Reports (PSURs)/Periodic Benefit-risk Evaluation Reports (PBRER)
Periodic safety update report (PSUR) provides a periodic and comprehensive assessment of the worldwide safety data of a marketed drug. Over time it was recognized that the risk of the marketed drug should be assessed in the light of its benefits and change in the risk estimate overtime. Consequently, the report name was changed to…