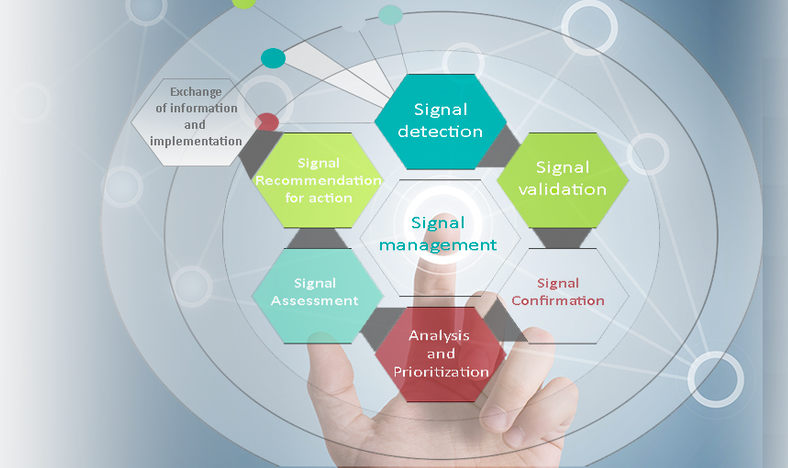
The objective of signal assessment is to further evaluate the significance and potential risk of a validated signal so as to identify the need for additional data collection, risk mitigation or minimization activities in a timely manner, or for any regulatory action.
The following steps will be performed for signal assessment:
- Review appropriate internal and external sources to obtain further information (e.g. literature articles, application dossier, spontaneous reports, expert consultation, or information held by regulatory authorities).
- Assess the significance of a signal at a broader level, at the therapeutic or at the System Organ Class (SOC) level, or at the level of a Standardized MedDRA Query (SMQ) to obtain a potential link to a complex disease, to a prior stage or a reaction or to clinical complications of the adverse reaction of interest.
- Document the risk assessment of the signal per product, and recommend no further action, or further action to prevent or minimize patient risk.
Based on the level of safety risk or patient impact, recommend action and review recommendations with the product safety board.